
7 min
5
10.04.2022

In everyday life, you come across a mixture. For example, you inhale carbon dioxide, nitrogen, and oxygen. Thus air is itself a widespread mixture. All substances can be divided into two types:
- Pure substance is a combination of elements of the same type. For example, elements such as copper and zinc are pure substances. The fact is that these substances consist of one component: a copper atom and a zinc atom. A pure substance contains a set of permanent elements. For example, the well-known water molecule (H2O) consists of the constant elements of hydrogen and oxygen;
- Impure substances are a chemical substance that consists of different types of particles. For example, a solution of sugar in water contains both water molecules and sugar molecules. Impure substance does not have a constant composition: the same mixture's constituents' content may be different.
Based on the above information, we can conclude that,
Mixture
Mixture — is a substance that includes more than two elements.
By connecting the two components, you should not get a chemical reaction. Under any circumstance, you should be able to separate the components of the mixture. Each element of the mixture has its own chemical identity. Usually, scientists choose the mechanical method to mix mixtures—for example, osmosis and diffusion.
When combining different elements, you can get unexpected physical properties that the chemical element did not appear before. For example, scientists from ancient Rome have found that if you mix water and alcohol, then both components during the reaction can have a different melting point and boiling point. However, when the components exist separately, their chemical properties and physical properties differ.
Mixture Examples in Everyday Life
Mixtures do not form as a result of chemical changes. Therefore, in life, you can often come across simple mixtures:
| saltwater | colloids | hydrocarbons |
| water and sand | suspensions | oil |
| salt and sugar | solutions | brass |
In everyday life, some compounds cannot be called mixtures:
- baking soda and vinegar;
- plasticine and glue to create slime;
- milk;
- ice cream;
- granite.
Types of Mixtures
Mixtures have different compositions and can be divided into two class:
Homogeneous Mixture
Homogenous mixtures are those in which, even with a microscope, it is impossible to detect particles of other substances. The uniform composition and physical properties in all parts of such a mixture are the same since there are no interfaces between its components. An excellent example of a homogeneous mixture is an alloy or compound of gases.
Ways to define a homogeneous mixture:
- All compounds are a homogeneous mixture.
- The particle size does not exceed one nanometer.
- You cannot apply the Tyndall effect.
- You cannot separate the borders of the elements.
- You cannot separate the constituent particles using centrifugation or decantation.
Heterogeneous Mixture
A heterogeneous mixture, or as they are called grainy, is a compound that consists of different substances that are not homogeneous or that have localized areas. You can find the individual substances that make up a heterogeneous mixture because they do not mix evenly. You can physically or chemically separate a heterogeneous mixture into its components. These mixtures always have more than one phase, and the composition varies from one region to another.
Ways to identify a heterogeneous mixture:
- Without a microscope, you can see that the mixture is heterogeneous.
- The constituent particles are unevenly distributed.
- You can distinguish all the ingredients that go into the mixture.
- The particle size reaches one micrometer.
- You can apply the Tyndall effect.
Homogeneous and heterogeneous mixtures are divided into several more types.
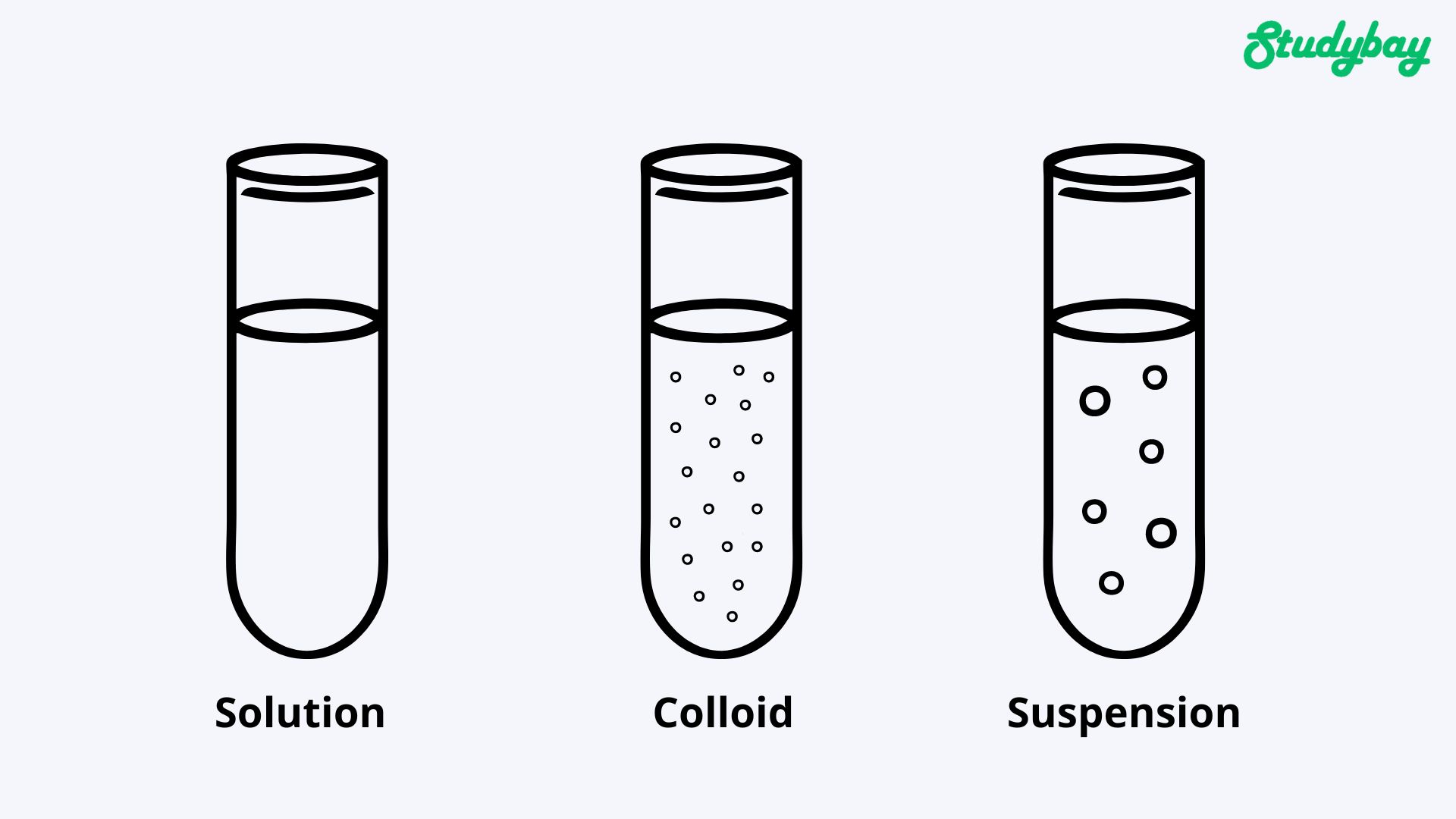
Solutions
Solutions are homogeneous systems consisting of particles of a solute, a solvent, and their interaction products. When working with solutions, scientists distribute the dissolved substance evenly in the solvent. The solution can consist of two or more components. Solutions are liquid, solid, and gaseous.
Colloids
Colloids are solutions of large molecules. They can provide a rapid increase in circulating blood volume. Also, colloids are associated with the development of allergic reactions, impaired coagulation, and renal failure development.
According to the type of internal structure, scientists distinguish three types of colloidal systems:
- Suspensions are solutions of metals and their compounds.
- Micellar solutions are solutions in which colloidal particles arise from the aggregation of amphiphilic molecules.
- Molecular these are solutions of natural or synthetic high molecular weight substances.
Suspensions
A suspension is a heterogeneous mixture where a solid is found in tiny particles in a liquid substance. Over time, any suspension stratifies under the influence of gravity. The solid settles to the bottom, for example, in a jar or vessel.
How to Prepare Mixture?
The process of making mixtures is different. In pharmaceutical factories, pharmacists maintain strict proportions, keep records, and record prescriptions. We will look at how to make a cough mixture at home:
Ingredients:
- One glass of milk.
- One spoonful of butter.
- One spoon of honey.
- One egg yolk.
- 1/4 teaspoon baking soda
To prepare an egg mixture, you must first boil a glass of milk. Then add a tablespoon of butter and honey. But also add the well-beaten egg yolk and just a little baking soda, about 1/4 teaspoon. It is a very effective remedy for coughs and bronchitis, laryngitis, and tracheitis.
As you can see, you don't have to be a pharmacist or an outstanding chemist to make a mixture. We hope our article helped you understand the mixtures. Based on the information, you will write an excellent essay, laboratory work, scientific project, presentation, or other student paper.



