7 min
0
10.04.2022

Chemistry belongs to the natural sciences and studies substances, their structure, properties, chemical compound, and their results, the laws by which all reactions and transformations operate. Since chemistry is a science unlimited, students have difficulty calculating atomic mass, molecular mass, number of moles. To show how to do the calculations quickly, we will look at the molar mass of benzene. However, the algorithm below can be applied to any substance.
Benzene - What Is This Substance?
Chemical formula - C6H6
Molecular formula - C6H6
Empirical formula - CH
Molar mass is 78,11 g/mol.
Atomic weight is 78,11 g/mol.
Number of atoms: benzene is a hexagonal molecule of six carbon atoms and six hydrogen atoms.
Boiling point is + - 80,1 °C.
Melting point is + - 5,5 °C.
Benzene (phenyl hydrogen) is an organic compound in an uncolored liquid with a slightly pungent sweet odor. Benzene is a hydrocarbon from a number of the simplest arenes. The attention of benzene is toxic, carcinogenic, and contaminant.
It is generally accepted that benzene is a component of gasoline. Many companies actively use it in various industrial sectors. For example, scientists create medicinal and veterinary drugs, a wide range of plastics, synthetic rubber, dyes, etc. Although this substance is present in crude oil, it is synthesized from its other industry components.
Physical Properties of Benzene
Benzene is a colorless, transparent, highly mobile liquid with aromatic compounds that burns with a bright smoky flame and forms flammable vapors. In the cold, it solidifies into a crystalline mass that melts at 6 ° C.
This compound is perfectly miscible with ether, chloride, cyclohexane, ethanol, methane, octane, oxide, toluene, xylene gasoline, and other organic solvents. Benzene is practically immiscible with water: no more than 0.08% dissolves at 22 ° C. Benzene is an excellent solvent for fats, resins, oils, asphalt, alkaloids, sulfur, phosphorus, iodine, etc. With some solvents, benzene forms azeotropic mixtures.
Chemical Properties of Benzene
We want to pay special attention to the chemical structure of benzene. Each of the six carbon atoms in its molecule is in a state of sp2 hybridization and is bonded to two adjacent carbon atoms and a hydrogen atom by three chemical structure -bonds. The bond angles between each pair of π-bonds are equal to 120 °. Thus, the skeleton of ð-bonds is a regular hexagon, in which all carbon atoms and all ð-bonds C-C and H-H lie in the same plane:
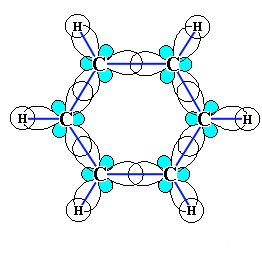
Substitution reactions are inherent in benzene. It reacts with nitrate acid, olefins, chloroalkanes, halogens, and sulfate acid. The rupture of the benzene ring is carried out at rigid parameters of temperature and pressure.
When interacting with olefins, substances homologous to benzene are formed, in particular ethylbenzene and isopropyl benzene. Reaction with Cl2 and Br2 in the presence of a catalyst component gives phenyl chloride. Other reactions typical of this reagent include formylation, sulfonation, nitration, catalytic hydrogenation, oxidation, ozonolysis.
How to Calculate the Molar Mass of Benzene?
It is effortless to calculate benzene molar mass. To do this, you can follow these steps:
- Prepare the periodic table in advance. You can find this table in any chemical book or open it on the Internet. With this table, you can write out the valencies of chemical elements and the atomic mass unit.
- The next step is to formulate a chemical formula. If you know what elements are included in the substance, you can draw up a formula using the periodic table. In our case, the elements form the following formula: C6H6.
-
Now it's time to start working with the periodic table. Open the table and write down each element's valence - C, H. You can also use the valence table to simplify the task. However, it is often not in chemistry books, so you will have to use the periodic table during the lesson.
- Having written out the valencies of each element, you can start calculating. However, before doing this, write down the weight of each chemical. The weight is in the periodic table. The atomic weight of C is 12.0107, and H is 1.00784. The formula also includes numbers that need to be multiplied along with the mass. You should get the following formulas:
Mr (C6H6) = 6 · Ar (C) + 6 · Ar (H).
Mr (C6H6) = 6 · 12 + 6 · 1 = 72 + 6 = 78. Based on these formulas, you can see that to calculate the molar mass of ethanol, you need to add all the masses together.
-
You are now close to the final step. Determine the mass of one molecule. To do this, you need to use the Avogadro number and the total molar mass of benzene. As a result, you can get the following formula:
m (C6H6) = Mr (C6H6) / NA = 78 / 6,02 · 1023 = 12,9 · 1023g
It is essential to end the task with these calculations so that the assignment looks complete.
Examples of Solving Tasks with Molar Mass of Benzene
In chemistry, you can find more complex tasks using the molar mass of benzene. Let's take a look at these tasks and examples of solutions.
Task 1
Task: Calculate the mass of benzene formed due to dehydrogenation of 184.8 g of cyclohexane.
Decision: First, we need to draw up the reaction equation - C6H14 = C6H6 + 4H2. Now we need to find ν of cyclohexane. We divide the mass by the molar mass: 184,8 / 86 = 2,15 mol.
Since the ratio of cyclohexane and benzene in the equation is the same, then benzene will also have ν equal to 2,15 mol. Then we find the mass of benzene, according to the formula. We need to multiply ν by the molar mass: 2,15 · 78 = 167,7g.
Answer: The mass of benzene is 167,7 g.
Task 2
Task: What mass of benzene (C6H6) was chlorinated in the light if 11,35 grams of hexachlorocyclohexane (C6H6Cl6) was obtained by chlorination of benzene which is 65% yield?
Decision: Let's write the reaction equation for benzene chlorination:
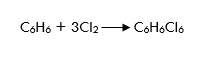
Recall that the reaction product's yield is understood as the ratio of the mass (number of moles) of the practically obtained substance to the mass theoretically calculated using the reaction equation:
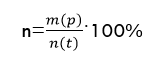
Let's calculate the theoretical mass of hexachlorocyclohexane (C6H6Cl6) by the formula:
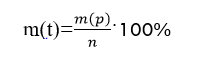
m (t) (C6H6) = 11,35 ⋅ 100/65 = 17,46 (g).
Taking into account that the molar mass of benzene (C6H6) and hexachlorocyclohexane (C6H6Cl6) is 78 g/mol and 220 g/mol, respectively, we find the mass of benzene (C6H6) using the reaction equation:
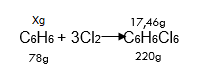
78 g of C6H6 participates in the formation of 220 g of C6H6Cl6
x g C6H6 participates in the formation of 17,46 g C6H6Cl6
From there, we get the following calculations:
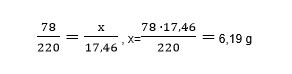
Consequently, 6,19 grams of benzene was chlorinated.
Answer: Chlorination of benzene weighing 6,19 grams.
How Is Benzene Mined?
The main ways to obtain pure benzene are:
- coking of coal raw materials;
- catalytic aromatization of petroleum gas fractions;
- pyrolysis of oil fractions (both gasoline and heavier);
- trimerization of ethine.
Where Is Benzene Used?
Currently, benzene is mainly used in the production of ethylbenzene, cumene, and cyclohexane. Based on benzene, various intermediate products are still obtained, which are used in the future to get synthetic rubbers, plastics, synthetic fibers, dyes, insecticides, medicinal substances, etc.
Scientists also use benzene to produce some insecticides, blowing agents, and flame retardants. People use benzene as a solvent and extractant to manufacture varnishes and paints or as a highly active additive to motor fuels.